Bioinspired Synthesis of (+)-Cinchonidine Using Cascade Reactions
Wentao Liu+, Wenfang Qin+, Xiaobei Wang, Fei Xue, Xiao-Yu Liu, and Yong Qin*
Angew. Chem. Int. Ed. 2018, 57 (38), 12299 –12302
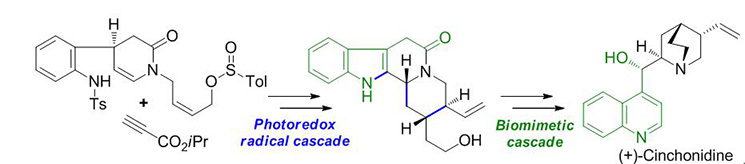
The development of efficient syntheses of complex natural products has long been a major challenge in synthetic chemistry. Designing cascade reactions and employing bioinspired transformations are an important and reliable means of achieving this goal. Presented here is a combination of these two strategies, which allow efficient asymmetric synthesis of the cinchona alkaloid (+)-cinchonidine. The key steps of this synthesis are a controllable, visible-light-induced photoredox radical cascade reaction to efficiently access the tetracyclic monoterpenoid indole alkaloid core, as well as a practical biomimetic cascade rearrangement for the indole to quinolone transformation. The use of stereoselective chemical transformations in this work makes it an efficient synthesis of (+)-cinchonidine.
Pre:Asymmetric Total Syntheses of the Akuammiline Alkaloids (-)-Strictamine and (-)-Rhazinoline
Recommend







