Asymmetric Total Syntheses of the Akuammiline Alkaloids (-)-Strictamine and (-)-Rhazinoline
Asymmetric Total Syntheses of the Akuammiline Alkaloids (−)‐Strictamine and (−)‐Rhazinoline
Wenfei Li+, Zhitao Chen+, Di Yu, Xin Peng, GuohuaWen, Siqi Wang, Fei Xue, Xiao-Yu Liu, and Yong Qin*
Angew. Chem. Int. Ed. 2018, 57 (22), 6667–6671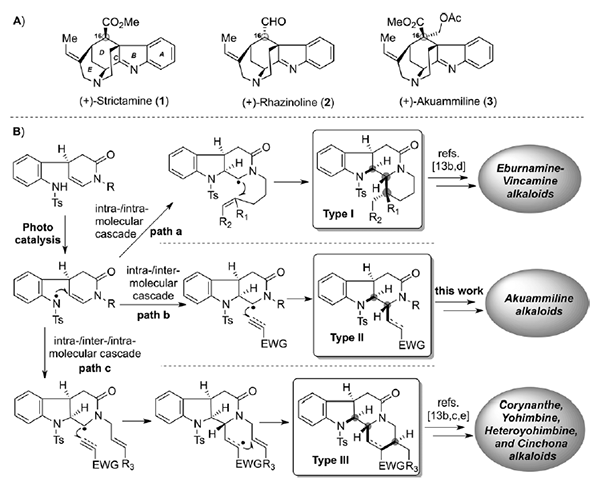
Strictamine and rhazinoline are representative methanoquinolizidine-containing akuammiline alkaloids that possess different stereochemistry at the C16 position. A unified approach to the enantioselective total syntheses of these two molecules is described. The key steps in this synthesis include a photocatalytic intra/intermolecular type II radical cascade reaction, a Tsuji–Trost allylation, a palladium- or nickelmediated cyclization, and a late-stage intramolecular N-alkylation reaction.
Pre:Enantioselective Total Synthesis of (−)-Arcutinine
Next:Bioinspired Synthesis of (+)-Cinchonidine Using Cascade Reactions
Recommend







