Enantioselective Total Synthesis of (−)-Arcutinine
Wei Nie,# Jing Gong,# Zhihao Chen, Jiazhen Liu, Di Tian, Hao Song, Xiao-Yu Liu, and Yong Qin*
J. Am. Chem. Soc. 2019, 141, 9712−9718
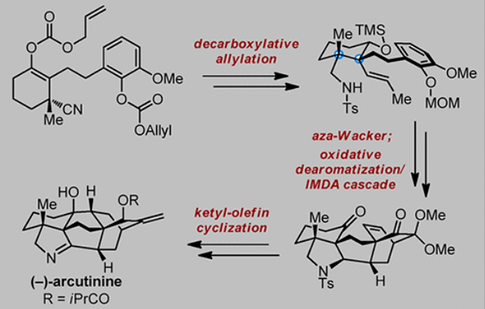
The first total synthesis of an arcutine-type C20-diterpenoid alkaloid arcutinine has been achieved in both racemic and asymmetric forms. Construction of the C4 quaternary center and the pyrrolidine E ring in an early stage proved to be important for achieving the successful synthesis of the target alkaloid. Strategically, an asymmetric conjugate addition/aldol cascade and a decarboxylative allylation reaction allowed the establishment of the vicinal all-carbon quaternary stereocenters at C4 and C5. Furthermore, a sequence consisting of an intramolecular aza-Wacker cyclization, an oxidative dearomatization/IMDA cascade, and a ketyl-olefin cyclization enabled the assembly of the core structure and led to the total synthesis of arcutinine.
Pre: Review: Indole Alkaloid Synthesis Facilitated by Photoredox Catalytic Radical Cascade Reactions
Next:Asymmetric Total Syntheses of the Akuammiline Alkaloids (-)-Strictamine and (-)-Rhazinoline
Recommend







