四川大学华西药学院
联系电话: 028-85503959
QQ:85106264
邮箱:haoright@163.com
复杂活性天然产物全合成
复杂结构的天然产物是自然界对人类分子创制能力预设的挑战,是有机合成化学发展的思想源泉和创新药物的重要来源,天然产物的全合成一直是有机合成化学研究领域最具挑战性和影响力的基础研究工作。本课题组针对复杂天然产物合成领域中高效构建天然产物骨架的核心科学问题进行研究,致力于发展创新合成策略。目前已发展以环丙烷化串联反应和氮自由基串联反应为代表的创新合成策略和方法,实现了部分类别天然产物的规模化制备,共完成了以结构最为复杂的吲哚生物碱和二萜生物碱为代表的70余个天然产物的全合成,其中21个天然产物的全合成为国际上首次合成,部分研究工作被评为全合成领域的突破性进展。
1. 基于环丙烷化-开环-亚胺环合的串联反应策略的天然产物全合成
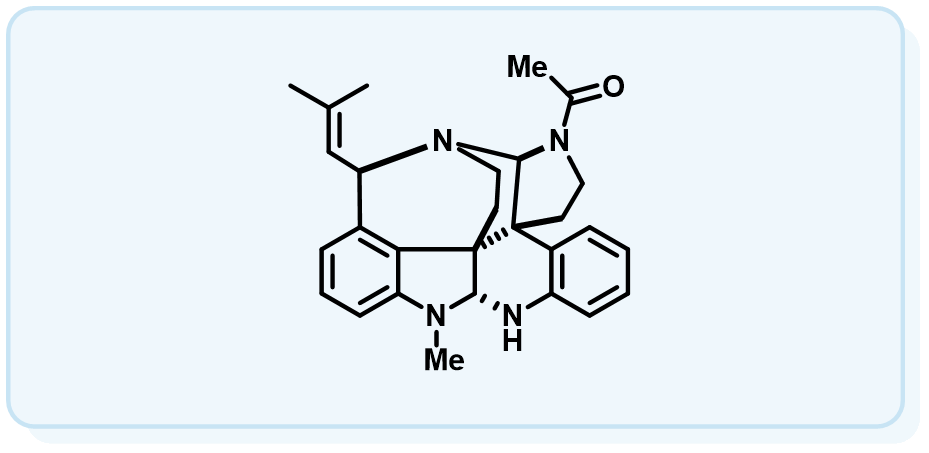
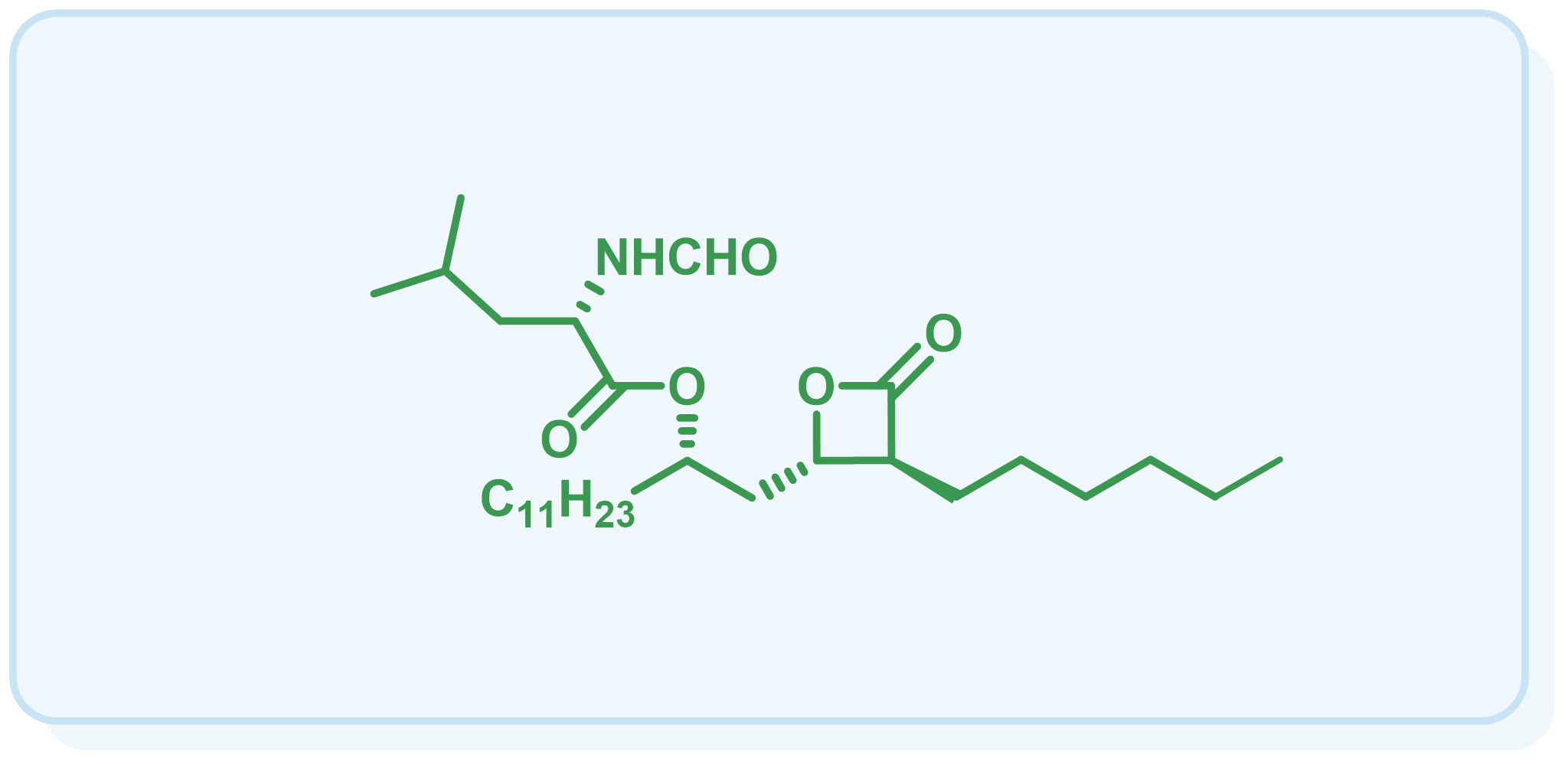
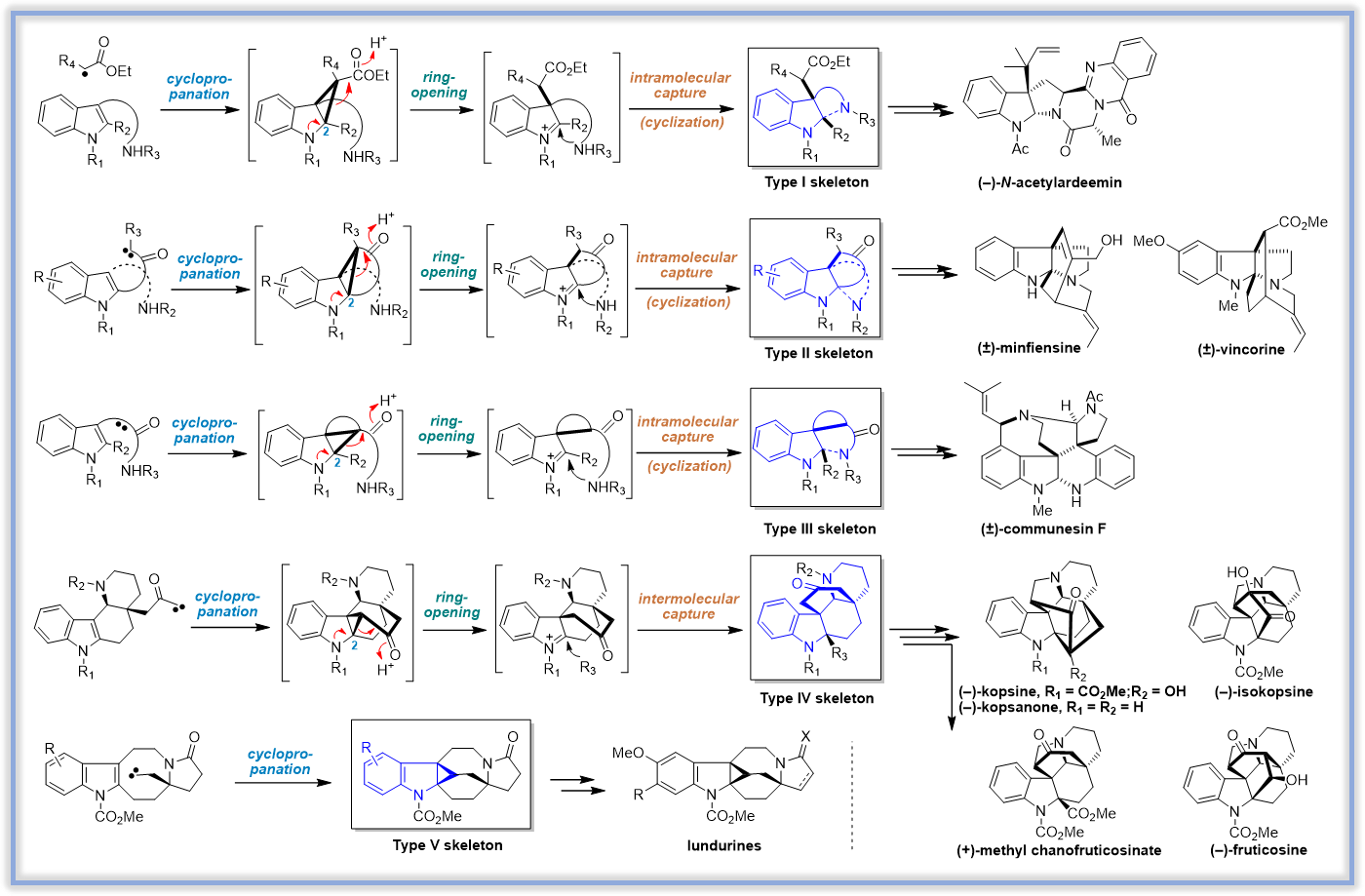
本课题组以具有重要药用价值的吲哚生物碱为研究对象,原创性地发展了一种基于环丙烷化-开环-亚胺环合的串联反应(CRI反应),建立了五种复杂吲哚生物碱骨架(Type I-Type V)的通用构建方法。应用该方法学,我们成功实现了一系列复杂吲哚生物碱的全合成。
相关论文:
(1) Biomimetic Approach to Perophoramidine and Communesin via an Intramolecular Cyclopropanation Reaction. Org. Lett. 2006, 8, 2187-2190.
(2)Total Synthesis of (±)-Communesin F. J. Am. Chem. Soc. 2007, 129, 13794.
(3)Synthesis of Chiral 3-Substituted Hexahydropyrroloindoline via Intermolecular Cyclopropanation. Org. Lett. 2006, 8, 6011-6014.
(4)Highly Efficient Assembly of Indole Alkaloid Skeleton via Cyclopropanation: Concise Total Synthesis of (±)-Minfiensine. Angew. Chem. Int. Ed. 2008, 47, 3618.
(5)Total Synthesis of (±)-Vincorine. J. Am. Chem. Soc. 2009, 131, 6013.
(6)Total Synthesis of (-)-Ardeemin. J. Org. Chem. 2009, 74, 298-304.
(7)Total Synthesis of Indoline Alkaloids: A Cyclopropanation Strategy. Acc. Chem. Res. 2011, 44, 447-457. (Review)
(8)Total Synthesis of (-)-Lundurine A and Determination of its Absolute Configuration. Angew. Chem. Int. Ed. 2015, 54, 2228-2231.
(9)Studies of a Diazo Cyclopropanation Strategy for the Total Synthesis of (-)‐Lundurine A. Chem. Eur. J. 2015, 21, 13284-13290.
(10)Asymmetric Total Syntheses of Kopsia Indole Alkaloids. Angew. Chem. Int. Ed. 2017, 56, 3703-3707.
(2)Total Synthesis of (±)-Communesin F. J. Am. Chem. Soc. 2007, 129, 13794.
(3)Synthesis of Chiral 3-Substituted Hexahydropyrroloindoline via Intermolecular Cyclopropanation. Org. Lett. 2006, 8, 6011-6014.
(4)Highly Efficient Assembly of Indole Alkaloid Skeleton via Cyclopropanation: Concise Total Synthesis of (±)-Minfiensine. Angew. Chem. Int. Ed. 2008, 47, 3618.
(5)Total Synthesis of (±)-Vincorine. J. Am. Chem. Soc. 2009, 131, 6013.
(6)Total Synthesis of (-)-Ardeemin. J. Org. Chem. 2009, 74, 298-304.
(7)Total Synthesis of Indoline Alkaloids: A Cyclopropanation Strategy. Acc. Chem. Res. 2011, 44, 447-457. (Review)
(8)Total Synthesis of (-)-Lundurine A and Determination of its Absolute Configuration. Angew. Chem. Int. Ed. 2015, 54, 2228-2231.
(9)Studies of a Diazo Cyclopropanation Strategy for the Total Synthesis of (-)‐Lundurine A. Chem. Eur. J. 2015, 21, 13284-13290.
(10)Asymmetric Total Syntheses of Kopsia Indole Alkaloids. Angew. Chem. Int. Ed. 2017, 56, 3703-3707.
2. 基于可见光催化产生氮自由基策略的天然产物全合成
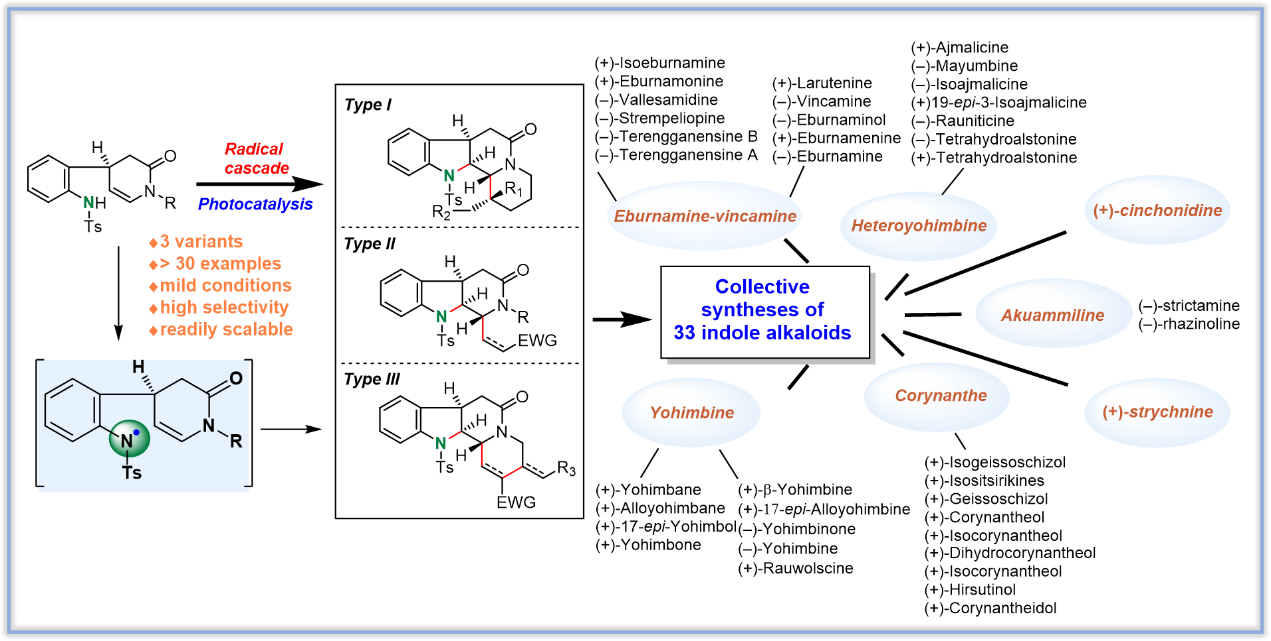
本课题组创新性地发展了在可见光催化条件下,通过去质子化和氧化将苯胺N-H官能基转化为氮自由基,实现了该缺电子氮自由基分子内加成到富电性的烯胺β碳原子上,并进一步引发分子内/分子内、分子内/分子间和分子内/分子间/分子内的三种类型的自由基串联反应,一锅多步地高效合成了如图所示的白坚木型(Type I)、四氢咔波啉型(Type II)和柯楠因型(Type III)三种官能化的手性单萜吲哚生物碱骨架。运用该方法学,最终实现了33个分属于四个不同家族的单萜吲哚生物碱的高效集群式合成。
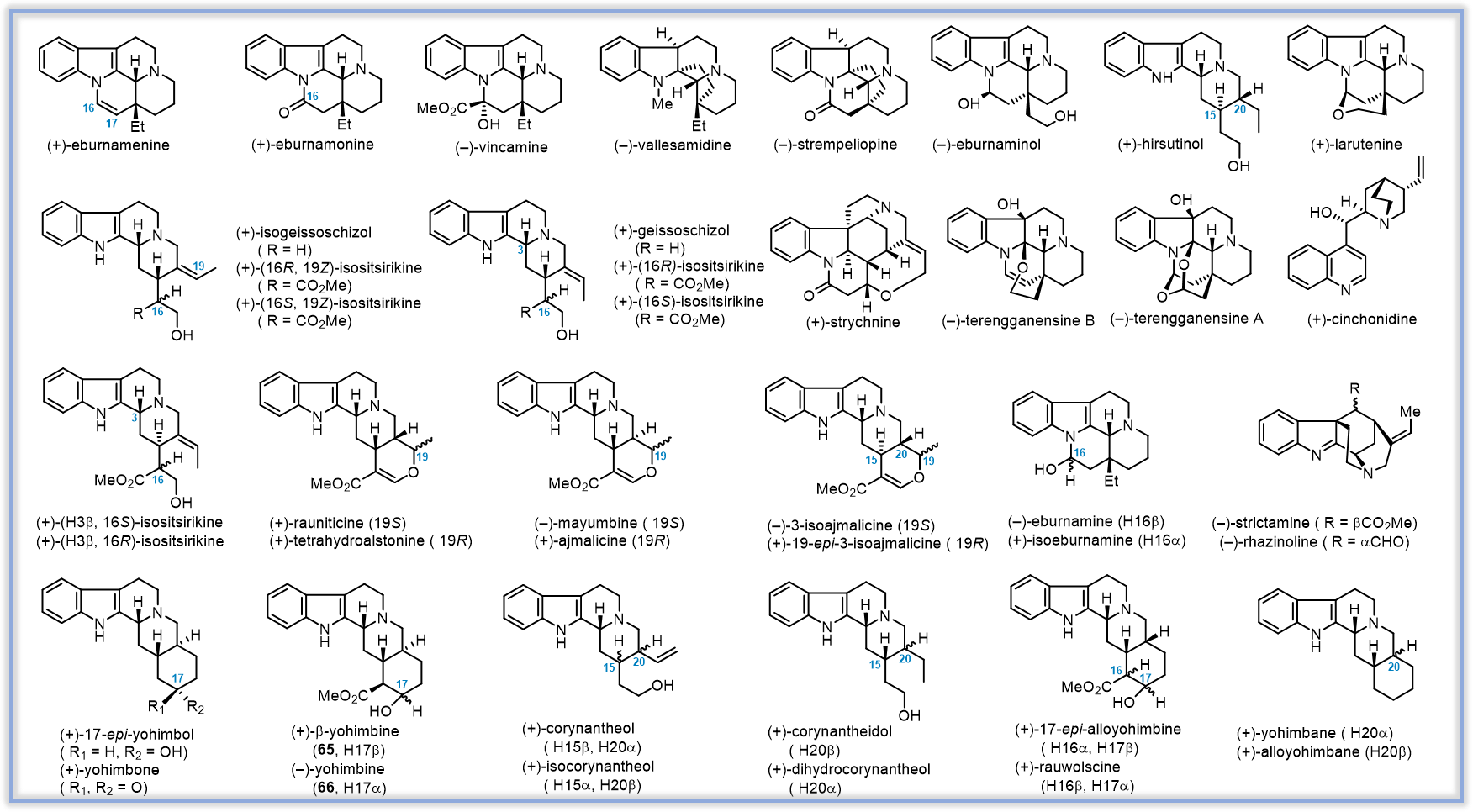
相关论文:
(1)A Radical Cascade Enabling Collective Syntheses of Natural Products. Chem, 2017, 2, 803-816.
(2)Bioinspired Synthesis of (+)‐Cinchonidine Using Cascade Reactions. Angew. Chem. Int. Ed. 2018, 57, 12299-12302.(1)A Radical Cascade Enabling Collective Syntheses of Natural Products. Chem, 2017, 2, 803-816.
(3)Concise syntheses of eburnane indole alkaloids. Chem. Commun. 2018, 54, 9510.
(4)Asymmetric Total Synthesis of (+)-Strychnine. Org. Lett. 2019, 21, 252-255.
(5)Asymmetric Total Syntheses of the Akuammiline Alkaloids (-)‐Strictamine and (-)‐Rhazinoline. Angew. Chem. Int. Ed. 2019, 58, 6059-6063.
(6) Indole Alkaloid Synthesis Facilitated by Photoredox Catalytic Radical Cascade Reactions. Acc. Chem. Res. 2019, 52, 1877–1891. (Review)
3. 二萜生物碱的全合成
本课题组针对具有复杂结构和广泛生理活性的二萜生物碱类天然产物,系统性地利用氧化去芳香化/Diels-Alder环加成关键反应策略,实现了多个来源于乌头属的二萜生物碱及相应的二萜分子的全合成。
本课题组针对具有复杂结构和广泛生理活性的二萜生物碱类天然产物,系统性地利用氧化去芳香化/Diels-Alder环加成关键反应策略,实现了多个来源于乌头属的二萜生物碱及相应的二萜分子的全合成。
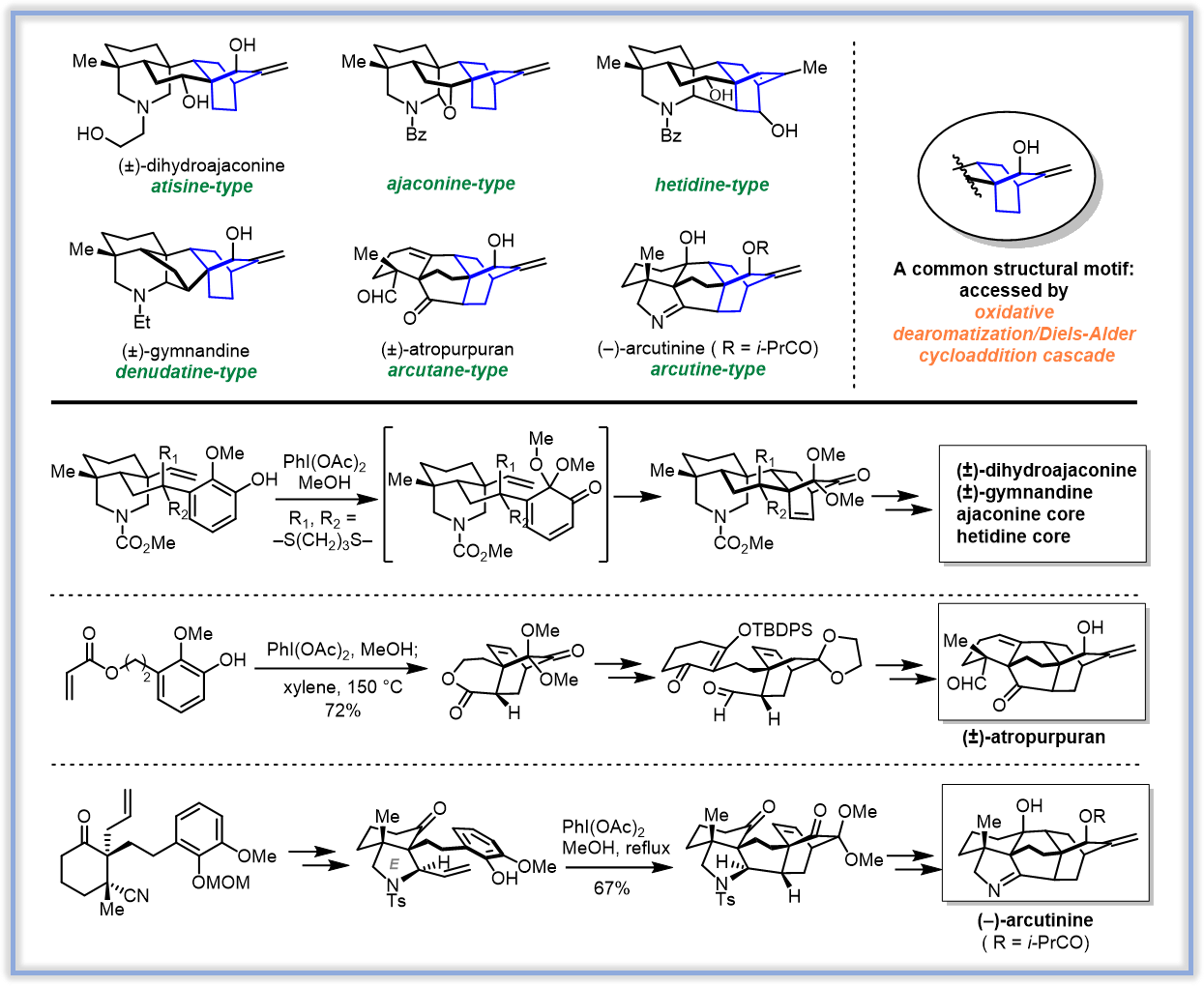
(3)Enabling syntheses of diterpenoid alkaloids and related diterpenes by an oxidative dearomatization/Diels–Alder cycloaddition strategy. Nat. Prod. Rep., 2017, 34, 1044-1050. (Review)
(4)Enantioselective Total Synthesis of (-)-Arcutinine. J. Am. Chem. Soc. 2019, 141, 9712-9718.
(5)Total Synthesis of Liangshanone. Angew. Chem. Int. Ed. 2020, 59, 23609-23614.
(6)Synthesis of Three-Dimensionally Fascinating Diterpenoid Alkaloids and Related Diterpenes. Acc. Chem. Res. 2021, 54, 22–34. (Review)
4. 其他创新策略应用于复杂天然产物全合成

(1)Synthesis and absolute configuration of hormone α1. Nat Chem Biol. 2008, 4, 235–237.
(2)Total Synthesis of (±)-Marinopyrrole A and Its Library as Potential Antibiotic and Anticancer Agents. J. Comb. Chem. 2010, 12, 541–547.
(3)Total Synthesis of (+)-Perophoramidine and Determination of the Absolute Configuration. J. Am. Chem. Soc. 2010, 132, 14052–14054.
(2)Total Synthesis of (±)-Marinopyrrole A and Its Library as Potential Antibiotic and Anticancer Agents. J. Comb. Chem. 2010, 12, 541–547.
(3)Total Synthesis of (+)-Perophoramidine and Determination of the Absolute Configuration. J. Am. Chem. Soc. 2010, 132, 14052–14054.
(4)Silver-promoted Friedel–Crafts reaction: concise total synthesis of (–)-ardeemin, (–)-cetylardeemin and (–)-formylardeemin. Org. Biomol. Chem., 2012,10, 2793-2797.
(5)Biomimetic Total Synthesis of (+)‐Gelsemine. Angew. Chem. Int. Ed. 2012, 51, 4909-4912.
(6)Total Syntheses of Ainsliadimer B and Gochnatiolides A and B. Chem. Eur. J., 2013, 19, 4423-4427.
(7)Total synthesis of (−)-depyranoversicolamide B. Chem. Commun. 2015, 51, 16143-16146.
(8) One-Pot Synthesis of Multisubstituted Butyrolactonimidates: Total Synthesis of (−)-Nephrosteranic Acid.
J. Org. Chem. 2015, 80, 2494-2502.
(9)Total Syntheses of (−)‐Isoschizogamine and (−)‐2‐Hydroxyisoschizogamine. Chem. Eur. J. 2015, 21, 14602-14607.
(10) Formal Synthesis of Anticoagulant Drug Fondaparinux Sodium. J. Org. Chem. 2016, 81, 162-184.
(11) Formal total synthesis of the akuammiline alkaloid (+)-strictamine. Chem. Commun. 2017, 53, 12665-12667.
(12) Formal Total Syntheses of (−)- and (+)-Actinophyllic Acid. J. Org. Chem. 2018, 83, 754-764.
(13) Total synthesis of akuammiline alkaloid (+)-strictamine. Tetrahedron 2018, 74, 1129-1134.
(14) Enantioselective Synthesis of ABCF Tetracyclic Framework of Daphniphyllum Alkaloid Calyciphylline N. Org. Lett. 2018, 20, 5053-5057.
(5)Biomimetic Total Synthesis of (+)‐Gelsemine. Angew. Chem. Int. Ed. 2012, 51, 4909-4912.
(6)Total Syntheses of Ainsliadimer B and Gochnatiolides A and B. Chem. Eur. J., 2013, 19, 4423-4427.
(7)Total synthesis of (−)-depyranoversicolamide B. Chem. Commun. 2015, 51, 16143-16146.
(8) One-Pot Synthesis of Multisubstituted Butyrolactonimidates: Total Synthesis of (−)-Nephrosteranic Acid.
J. Org. Chem. 2015, 80, 2494-2502.
(9)Total Syntheses of (−)‐Isoschizogamine and (−)‐2‐Hydroxyisoschizogamine. Chem. Eur. J. 2015, 21, 14602-14607.
(10) Formal Synthesis of Anticoagulant Drug Fondaparinux Sodium. J. Org. Chem. 2016, 81, 162-184.
(11) Formal total synthesis of the akuammiline alkaloid (+)-strictamine. Chem. Commun. 2017, 53, 12665-12667.
(12) Formal Total Syntheses of (−)- and (+)-Actinophyllic Acid. J. Org. Chem. 2018, 83, 754-764.
(13) Total synthesis of akuammiline alkaloid (+)-strictamine. Tetrahedron 2018, 74, 1129-1134.
(14) Enantioselective Synthesis of ABCF Tetracyclic Framework of Daphniphyllum Alkaloid Calyciphylline N. Org. Lett. 2018, 20, 5053-5057.
(15) Asymmetric Synthesis of an Advanced Tetracyclic Framework of (+)-Sarain A. Org. Lett. 2018, 20, 6701-6704.
(16) Progress towards the synthesis of aconitine: construction of the AE fragment and attempts to
access the pentacyclic core. Org. Chem. Front. 2019, 6, 377–382.
(17) Construction of the highly oxidized bicyclo[3.2.1]octane CD ring system of aconitine via a late stage enyne cycloisomerization. Chem. Commun. 2018, 54, 12258-12261.
(18) Practical synthesis of immucillins BCX-1777 and BCX-4430. Org. Chem. Front., 2020, 7, 3675-3680.
(19) Bioinspired scalable total synthesis of opioids. CCS Chemistry, 2021, accepted.
(16) Progress towards the synthesis of aconitine: construction of the AE fragment and attempts to
access the pentacyclic core. Org. Chem. Front. 2019, 6, 377–382.
(17) Construction of the highly oxidized bicyclo[3.2.1]octane CD ring system of aconitine via a late stage enyne cycloisomerization. Chem. Commun. 2018, 54, 12258-12261.
(18) Practical synthesis of immucillins BCX-1777 and BCX-4430. Org. Chem. Front., 2020, 7, 3675-3680.
(19) Bioinspired scalable total synthesis of opioids. CCS Chemistry, 2021, accepted.
上一篇:第一页
下一篇:先进药物制造
相关推荐